
We shall discuss about these, in detail, in our later posts.
#ELEMENTS TABLE WITH ATOMIC MASS AND ATOMIC NUMBER HOW TO#
( NOTE – How to remember this? Alphabetically A is always at the top and Z at the bottom.Similarly, the mass number(A) is written at the top and the atomic number (Z) at the bottom). The atomic number(Z) is written as a subscript and atomic mass number(A) is written as superscript, to the left of the element symbol. This quantity gives us the number of protons and neutrons in the nucleus of an atom.As the atomic number increases with each element in the periodic table, so does the atomic mass number. As seen above the three different isotopes of hydrogen have the same atomic number but their atomic mass number distinguishes them from one another.
:max_bytes(150000):strip_icc()/PeriodicTableSigFigBW-58b5c7f25f9b586046cae098.png)
Atomic number gives an element its chemical identity. Atomic number is the most fundamental property, as it dictates the properties an element possess.

We have already studied that ‘the properties of elements are periodic functions of their atomic numbers’. The elements are arranged in increasing order of their atomic numbers in the periodic table. It can also be defined as the number of electrons in a neutral atom.Į.g.– Atomic number of hydrogen is Z = 1, as it contains only 1 proton.Deuterium, tritium which are isotopes of hydrogen also have the same atomic number as the number of protons in their nucleus remains the same (although number of neutrons is different).
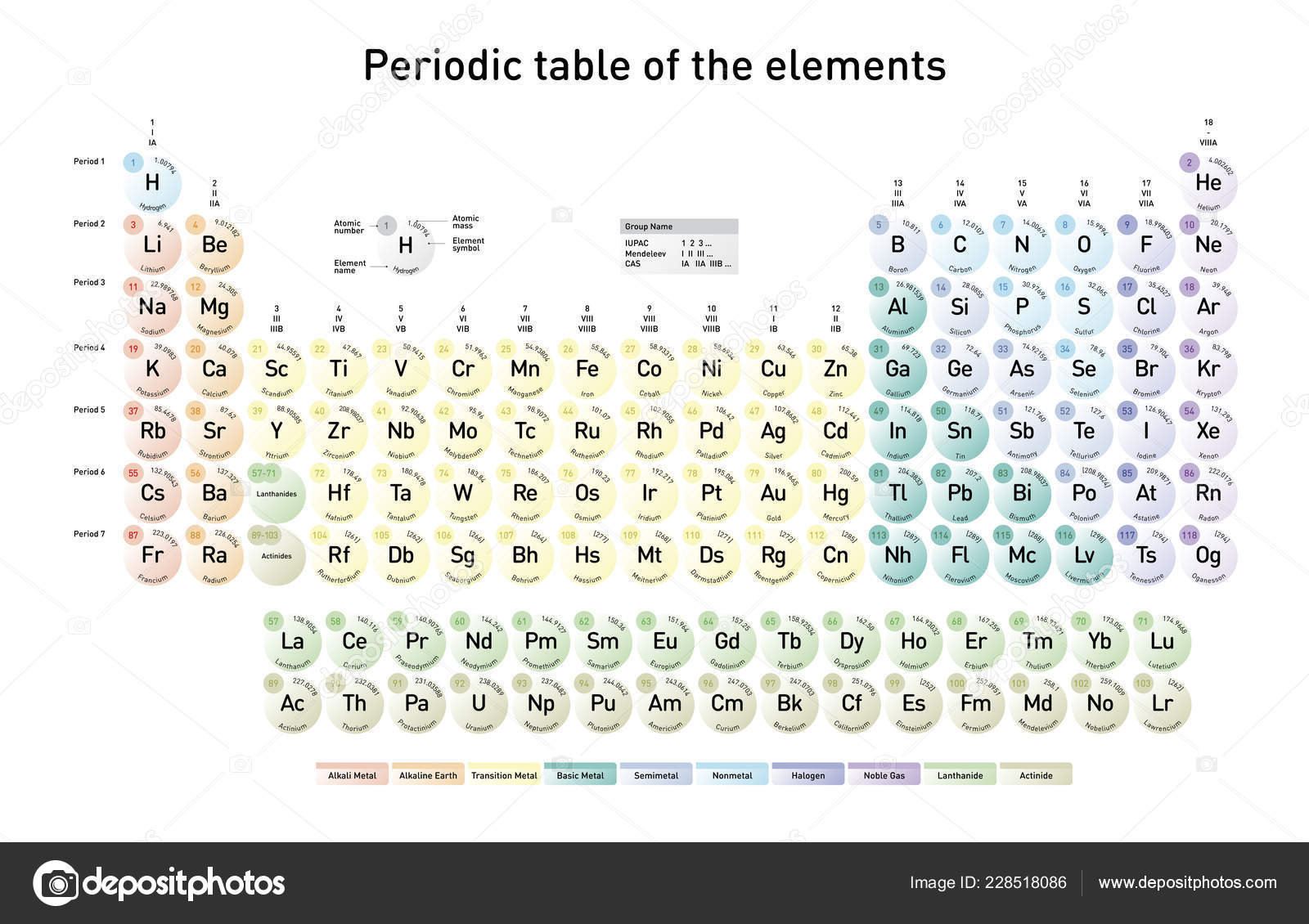
The arrangement of elements is such that their properties show a periodic trend across a row and down a group.Let us start studying the various properties of elements, which gives the arrangement of elements in the periodic table a beautiful pattern.Ītomic number (Z) is the number of protons found in the nucleus of an element. We shall begin our discussion on the general periodic trends of the periodic table from this post onwards.Periodicity means the tendency to recur/repeat at regular intervals.The table of elements manifests this phenomenon in many ways.


 0 kommentar(er)
0 kommentar(er)
